INTRODUCTION
The C5 inhibitor eculizumab is the current standard of care for the treatment of PNH. However, some pts receiving eculizumab to treat PNH continue to experience ongoing hemolysis and anemia, resulting in red blood cell transfusion dependence, substantial unmet clinical needs, and considerable health economic burden. This study evaluated the treatment patterns of pts with PNH receiving eculizumab and compared HRU among blood transfusion-dependent (TD) pts versus blood transfusion-free (TF) pts in real-world clinical practice in the United States.
METHODS
Pts aged ≥12 years with ≥2 claims for eculizumab infusion between April 1, 2014, and September 30, 2019, were identified from the IBM® MarketScan® Research Databases. The index date was the first observed claim for eculizumab infusion with ≥3 months of continuous eligibility prior (baseline period). Pts with ≥1 diagnosis of indications other than PNH for eculizumab (ie, atypical hemolytic uremic syndrome, generalized myasthenia gravis, neuromyelitis optica spectrum disorder) during the baseline period or on the index date were excluded to identify pts with PNH. The full cohort of PNH pts treated with eculizumab were then stratified into the TD cohort (≥1 claim for blood transfusion within 6 months following any eculizumab infusion) or TF cohort. Patient demographic and clinical characteristics in the baseline period were compared between TD and TF cohorts using standardized differences (std diff), with >20% indicating substantial differences. Treatment patterns and HRU (all-cause and PNH-related) were evaluated during the observation period (ie, from index date to earliest date between end of continuous healthcare plan enrollment and end of data availability). Time from index date to treatment discontinuation among pts in the full cohort was assessed using Kaplan-Meier analysis. HRU was compared between TD and TF cohorts using incidence rate ratios (IRRs) adjusted for baseline covariates.
RESULTS
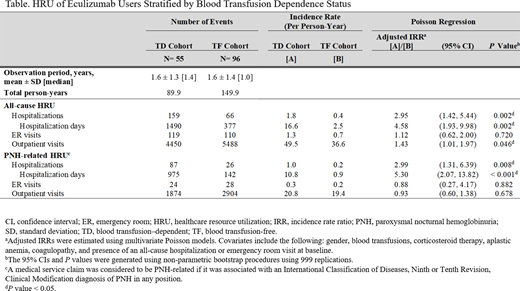
A total of 151 eculizumab users with PNH were identified as the full cohort; 55 (36%) were TD and 96 (64%) were TF. Mean (range) age was 36.7 (12-74) years among all pts (TD: 35.1 years; TF: 37.6 years). Overall, 56% of pts were female, with a higher proportion in the TD vs TF cohort (67% vs 49%; std diff = 38%). More pts in the TD vs TF cohort had blood transfusions (71% vs 18%; std diff = 127%) and use of corticosteroid therapy (46% vs 32%; std diff = 27%) during baseline. Baseline coagulopathy and aplastic anemia were more common in the TD vs TF cohort (coagulopathy: 49% vs 30%; std diff = 39%; aplastic anemia: 64% vs 43%; std diff = 43%). Myelodysplastic syndrome was present in 10% of the full cohort (TD: 13%; TF: 8%; std diff = 14%). During a mean observation period of 19 months for the overall sample (TD: 20 months; TF: 19 months), pts had a median (interquartile range) of 8 (3-30) eculizumab infusions (TD: 5 infusions; TF: 8 infusions) during maintenance phase; 29% of pts (TD: 21%; TF: 33%) had on average 14 days between maintenance eculizumab infusions; 61% of pts (TD: 66%; TF: 58%) discontinued eculizumab treatment (ie, gap of >42 days between 2 infusions) and the median time to treatment discontinuation was 254 days (TD: 180 days; TF: 337 days). Mean (range) number of blood transfusions among TD pts was 8.5 (1-54). During the observation period, pts in the TD cohort had 2.95 times more all-cause hospitalizations and 4.58 times more hospitalization days compared to pts in the TF cohort (all P < 0.05). Similar trends were observed for PNH-related hospitalizations. Results for all-cause and PNH-related HRU are presented in the Table.
CONCLUSIONS
This study demonstrated a considerable economic burden among pts with PNH treated with eculizumab, particularly among pts dependent on blood transfusions. TD eculizumab users comprised >36% of the overall sample, indicating that disease activity may not be well controlled. These pts had >4 times the number of hospitalization days compared to TF pts, emphasizing the substantial unmet clinical need despite treatment with eculizumab. These findings suggest that the current PNH standard of care may be insufficient for TD pts. Based on time between dosing intervals, >70% of PNH pts in this study were not dosed per label, and two-thirds of pts discontinued eculizumab within an average of a 1.5-year timeframe. New therapies are needed to reduce the considerable burden of pts with PNH.
Cheng:Apellis: Research Funding. Sarda:Apellis: Current Employment, Current equity holder in publicly-traded company. Mody-Patel:Apellis: Current Employment, Current equity holder in publicly-traded company. Krishnan:Apellis: Current Employment, Current equity holder in publicly-traded company. Yenikomshian:Apellis: Research Funding. Scoble:Apellis: Current Employment, Current equity holder in publicly-traded company. Mahendran:Apellis: Research Funding. Lejeune:Apellis: Research Funding. Yu:Apellis: Research Funding. Duh:Apellis: Research Funding; Takeda Oncology: Research Funding; GlaxoSmithKline: Research Funding; AstraZeneca: Research Funding; Blueprint Medicine: Research Funding; Novartis: Research Funding; Shire: Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal